Liquid Drug Filling: Miniature Batches Bring Changes to Pharmaceutical Processes
2023-10-06
Higher, further, faster – this principle has long been considered sacred and inviolable, particularly in the medical field. For decades, mass production of drugs has been the production standard, but today, individualized therapies are playing an increasingly important role in treating diseases. These very expensive drugs often require substantial investment in research and development, as well as in the concept of new machines, which need to be able to adjust to changing demands in order to flexibly produce small and micro batches of drugs.
The pharmaceutical industry is experiencing unprecedented change. Ten years ago, blockbuster drugs produced on a mass scale, along with traditional drugs, were the focus for many research and manufacturing companies. Today, these drugs continue to make a significant contribution to human health. Blood thinners, pain relievers, and insulin, for example, have become an essential part of the pharmacopeia. They can be produced continuously 24 hours a day on production lines up to 30 meters long. Thanks to advanced processes and packaging technology, this production is still secure for the foreseeable future.
From Blockbuster Drugs to Leading-edge ATMPs
For increasingly individualized disease treatment methods, traditional thinking needs to change. There are currently some specialized and often personalized drugs that can be used to cure or at least slow down some life-threatening diseases. Cell and molecular biotechnology have undergone a revolution along with pharmaceutical production. While high-speed production lines used to be the mainstream demand, now more and more pharmaceutical companies are investing in the research and development and commercialization of small batches of drugs, which are very different from the requirements for traditional drug production and filling. The growing prevalence of leading-edge therapies like cell and gene therapies and tissue engineered products, or Advanced Therapy Medicinal Products (ATMPs), indicates that the industry is steadily specializing. These drugs cannot be produced on large production lines. Instead, they focus on what is often referred to as small-batch production.
The ICH guidelines do not provide a precise definition of the term “small batch”. However, the US FDA provides an answer for generic injectables. According to the “Industry Guide, ANDAs: Questions and Answers about the Stability Tests of Active Pharmaceutical Ingredients and Formulations”, a small batch is at least 10% of the proposed maximum commercial batch, or less than 15,000 to 60,000 containers (depending on the fill volume)1. The batch sizes of injectable drugs used for clinical trials are usually reduced to a few thousand doses. For highly customized drugs, such as autologous cell therapies, the batch size is even smaller. On average, a patient needs about five to ten doses.
Highest Flexibility, Lowest Rejection Rates
The number of cell and gene therapies in development is increasing, highlighting the importance of ATMPs. Traditional high-speed machines can no longer meet the requirements of these new products. But how can these small and even micro batches be economically produced? What kind of production line will meet the needs of pharmaceutical companies and patients? Flexibility is the first factor.
Different products need to be filled into different sizes and types of containers, such as vials, syringes or cartridges. In handling and producing these biotech drugs in a flexible manner, disposable technologies are especially popular because they avoid the work and costs associated with cleaning validation. Another standard for flexibility is to reduce the number of change parts, thus avoiding lengthy changeovers. In addition, these small-batch demands maximize product recovery or minimize rejection rates. In short, any loss of product must be avoided.
Automation ensures higher safety
Human intervention is still a major cause of drug contamination. Automation is crucial in reducing or eliminating these interventions. For example, as early as 2004, the US FDA required that “equipment used in sterile production should be designed to limit the number and complexity of aseptic interventions by personnel.” In addition, the US FDA states, “Automation of other process steps, including the use of robots and other technologies, can further reduce the risk to the product.” 2 In the long term, aseptic filling will progress from being human-centered to fully automated.

Human intervention is still a major cause of drug contamination. Automation is crucial in reducing or eliminating these interventions.
This change is fully underway. For example, if containers are transferred to the filling station and stoppering station in the isolator by a robotic arm, the risk of contamination is significantly reduced. Robots can also reduce format parts to avoid glass-to-glass contact. Newly developed systems, such as Syntegon’s Versynta FFP (Flexible Filling Platform), also use laminar flow optimized design to ensure uninterrupted air flow to the containers and form single-direction flow protection. 100% In-Process Control (IPC) during filling minimizes product loss and ensures that virtually every drop of high-quality product is filled.

Syntegon offers the Versynta FFP product portfolio, which is standardized, highly modularized and has short delivery times, for pharmaceutical manufacturers, R&D labs, and biotech start-ups.
Glove-Free Production for Micro Batches
Given the above understanding of small batches, the production scale may be further reduced by orders of magnitude. With the Versynta FFP modular system, the capacity of the equipment is 1h when the comparable product has a production rate of 120 to 500 containers per hour compared to a maximum of 3600 vials, syringes, or cartridges per hour. The highly flexible, fully automatic Versynta microBatch production unit fills and seals different containers with minimal batch size. Batch changes can be completed within two hours. Nearly lossless filling of glass or plastic syringes, cartridges and vials can be realized.

The Versynta FFP can be easily adapted to different container types, such as vials, syringes and cartridges, as well as various filling systems including disposable solutions.
Not only is the output of this machine small, but its size is also very small. The machine is only 3.5 meters long, approximately 2 meters wide and 3 meters high, and can easily be integrated into an existing production environment. The size of the isolator unit itself is only 1.6 x 1.5 meters. It includes a cartoning, 100% process-controlled filling station and a combination stoppering and capping station. With the inclusion of an integrated air handling unit, there are hardly any installation interfaces with the building or technical gallery. New developments are setting new standards, especially in the area of automation. Gloveless isolators with integrated air handling significantly reduce the risk of contamination by eliminating operator manual intervention. The microBatch setup meets the highest aseptic requirements as outlined in Annex 1. Steam sterilizable items are passed through an interface and installed by a robot.

The Versynta microBatch is a highly flexible and fully automatic glove-free production unit for filling and sealing liquid (biological) medications.
Rising trend in RTU containers
The latest developments in the field of small micro batches correspond to another trend. The market for Ready-To-Use (RTU) containers has been growing rapidly for years. This is not surprising, as pharmaceutical companies benefit from simpler production processes, lower total costs, and higher flexibility. RTU containers are the preferred choice for small batch production, and their numbers are steadily growing. According to a recent report3, the global market for RTU vials alone is expected to reach $1.18 billion in the next ten years, predicting a growth rate of 14.5%.
Apart from vials, pre-sterilized cartridges are also gaining popularity. However, RTU syringes paved the way for other pre-sterilized containers as early as the 1980s. In fact, the advantages of small-batch RTU containers are considerable. For example, bulk syringes are supplied non-sterile. Because they cannot stand stably, they require complex handling on the machine and during filling. The syringes also need to be siliconized, which involves a special process dependent on change parts. The latter depends on the way the syringe is filled. For example, biologics require particularly low levels of siliconization.
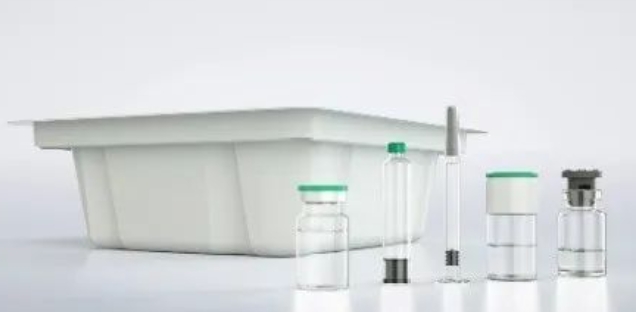
The market for ready-to-use containers (RTU) has been growing rapidly for years, particularly in small batch production.

RTU containers were first used on syringes and have paved the way for other pre-sterilized containers since the 1980s.
Saving Time, Space, and Cost
The benefits of RTU containers become particularly evident in the process stages mentioned above. Although they are still cost-intensive at present, they save pharmaceutical manufacturers a lot of time, space, and cost. Many steps, such as cleaning of parts, siliconization, and sterilization, are taken over by the RTU container packaging suppliers. They have the expertise and ensure that all processes meet the current global requirements and are validated, and that certified endotoxin, bacterial and particle concentration reports are delivered with the containers. For pre-sterilized syringe systems, this also includes the necessary siliconization process.
With the steady growth in demand for high-priced ATMPs and a variety of RTU packaging materials, there is likewise a steady growth in demand for new equipment for small and micro batches. The latter not only need to be more flexible in handling packaging materials, but also need to meet the trend toward automation and as little human intervention as possible. Ideally, primary packaging material and machine manufacturers should work closely together to jointly develop new solutions. Only when both partners not only follow trends, but actively innovate in system development, can pharmaceutical manufacturers ensure that they produce the highest level of safety for patients.




